Structure of an atom. An atom is electrically neutral as the protons and electrons are equal in magnitude.
 Atomic Structure Frcr Physics Notes
Atomic Structure Frcr Physics Notes
The protons and neutrons make up the nucleus of the atom which is surrounded by the electrons belonging to the atom.
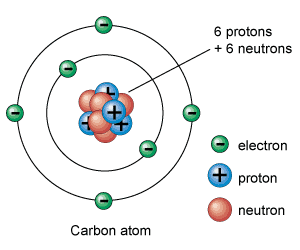
Structure of an atom. Atoms consist of a nucleus made of protons and neutrons orbited by electrons. The hydrogen atom H contains only one proton one electron and no neutrons. This can be determined using the atomic number and the mass number of the element see the concept on atomic numbers and mass numbers.
These electrons orbit the nucleus. It constitutes positively charged particles protons and uncharged particles neutrons Negatively charged particles called electrons revolve in orbit around the nucleus. The way these electrons are positioned is known as the electron configuration.
Each of these parts has an associated charge. They are so small that accurately predicting their behavior using classical physicsas if they were tennis balls for exampleis not possible due to quantum effects. Protons electrons and neutrons.
Any such particle that revolves would undergo acceleration and radiate energy. The structure of an atom is a positively charged sphere that embeds electrons in it. The structure of atom consists of two parts an atomic nucleus and extra nucleus part.
Protons and neutrons are found in the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic. Atomic structure - AQA Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells.
The nucleus is the positively charged centre of an atom and contains most of its mass. Atoms are extremely small typically around 100 picometers across. An atom is made of three parts protons neutrons and electrons.
An atom is the smallest unit of ordinary matter that forms a chemical elementEvery solid liquid gas and plasma is composed of neutral or ionized atoms. The tiny atomic nucleus is the center of an atom. It is composed of protons which have a positive charge and neutrons which have no charge.
The atomic number of an element describes the total number of protons in its nucleus. Let us now take a closer look at the microscopic structure of the atom what the atom looks like inside. Thomson was the first one to propose a model for the structure of an atom.
Atoms are made up of. Atoms consist of three basic particles. Thomsons structure of an atom failed to explain the arrangement of protons and electrons in its structure.
The protons are placed in the nucleus of the atom while the electrons are situated in the orbital shell of the atom. Electrons are the smallest of the three particles that make up atoms. For explaining this many scientists proposed various atomic models.
The protons carry a positive charge electrons have a negative charge and neutron possess no charge. Extra nuclear part means the space around the nucleus in which the electrons are distributed. Elements such as helium depicted here are made up of atoms.
An atom is made up of two parts nucleus and extra nuclear part. 421 THOMSONS MODEL OF AN ATOM Thomson proposed the model of an atom to be similar to that of a Christmas pudding. So far we have discussed that atoms are made up of a positively charged nucleus surrounded by one or more negatively charged electrons.
In this video we cover the structure of atoms what are subatomic particles energy levels and stable and reactive atomsTranscript and notesAtomic structur. Nucleus is the center of the atom with positive charge. Electrons are found in shells or orbitals that surround the nucleus of an atom.
The electrons in a sphere of positive charge. The nucleus center of the atom contains the protons positively charged and the neutrons no charge. Primarily the atomic structure of matter is made up of protons electrons and neutrons.
The outermost regions of the atom are called electron shells and contain the electrons negatively charged. As noted in the introduction to this article an atom consists largely of empty space. They group together in the center of the atom.
Drawbacks of Thomsons Model. The revolving electron would lose its energy and finally fall into the nucleus the atom would be highly unstable. According to Rutherfords model of an atom the electrons are revolving in a circular orbit around the nucleus.
The whole mass of the atom is located in the nucleus. Each shell can hold a certain amount of electrons before moving onto the next shell.