Likewise a non-metal becomes stable by gaining electrons to complete its valence shell and become negatively charged. Force that holds groups of two or more atoms together and makes the atoms function as a unit.
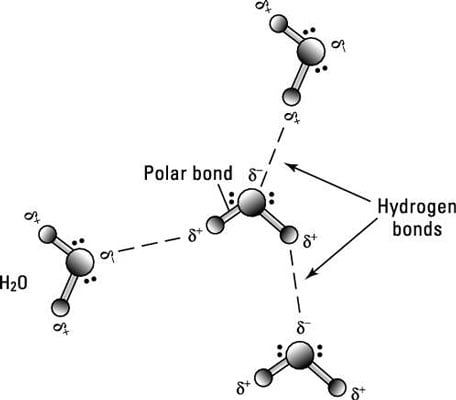 4 Types Of Chemical Bonds Dummies
4 Types Of Chemical Bonds Dummies
Secondary weak bonds This class includes the weaker more temporary bonds like London dispersion forces dipole-dipole force and hydrogen bonding.

Types of bonds chemistry. Bond in which one or more electrons from one atom are removed and attached to another atom resulting in positive and negative ions which attract each other. Chemistry Types of Bonds. A single bond forms with only one shared pair of electrons Notice that for many simple examples of sharing the number of lone electrons.
By definition a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. There are 4 primary types of chemical bonds which are formed by atoms or molecules to yield compounds. Chemical bonds Ionic bond.
Other types of bonds include metallic bondsand hydrogen bonding. For oxygen and nitrogen the atoms must share more than one electron to reach an octet for each atom. Chemical bonds include covalent polar covalent and ionic bonds.
Covalent bondA type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. Other types include the double bond the triple bond one- and three-electron bonds the three-center two-electron bond and three-center four-electron bond.
Bonds are divided into Chemical bonds Ionic bond Covalent bond Coordinate bond and Physical bonds Hydrogen bond Metallic bond. The type of chemical bonds formed vary in strength and properties. Intramolecular force and potential energy.
If youre seeing this message it means were having trouble loading external resources on our website. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces. When two atoms share two pairs of electron then a double bond forms.
1 Ionic bond Ionic bonding involves a transfer of an electron so one atom gains an electron while one atom loses an electron. Atoms with large differences in electronegativity transfer electrons to form ions. These types of bonds in chemical bonding are formed from the loss gain or sharing of electrons between two atomsmolecules.
In this video we discuss how chemical bonds are formed we cover ionic bonds and covalent bonds. Bond length and bond energy Opens a modal Worked example. When two atoms share three pairs of electrons then a triple bond forms.
Atoms and ions bond with each other in three main ways ionic bonds covalent bonds and metallic bonds. Types of chemical bonds 1. Chemical bonds hold molecules together and create temporary connections that are essential to life.
In chemistry we refer to Chemical bonding as a means or a way by which an atom attaches itself with other atoms. A covalent bond is formed when atoms share valence electrons. Terms in this set 29 Types of Elements in a Covalent Bond.
Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds. Types of chemical bonds. In non-polar covalent bonds the electronegativity difference between the bonded atoms is small typically 0 to 03.
The attractive forces between molecules in a liquid can be characterized as van der Waals bonds. There are two main types and some secondary types of chemical bonds. The ions then are attracted to each other.
Ionic bonding is a type of chemical bonding which involves a transfer of electrons from. Atoms or ions are held together in molecules or compounds by chemical bonds. Chemical bond formed by the electrostatic attraction of ions EN diff 15.
AYOOB Types of chemical bond 2. All bonds can be classified into two types Primary strong bonds- These include the bonds which are strong and difficult to break like covalent ionic and metallic bonds. Different types of bonds form different types of structures lattices and molecules.
Main Types of Chemical Bonds The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Ionic bonds form when metals and non-metals chemically react. One of the resulting ions carries a negative charge anion and the other ion carries a positive charge cation.
Types of elements in an Ionic Bond. These types of chemical bonds include. Basically there are three types of chemical bonding in chemistry and they are covalent bonding ionic bonding and metallic bondingIn this IGCSE chemistry chemical bonding blog post I am going to cover the basic concepts of these three types of bonding.
Interpreting potential energy curves of diatomic molecules Opens a modal Lattice energy Opens a modal Ionic bonds and Coulombs law Opens a modal Practice. Nonmetals can form different types of bonds depending on their partner atoms. Chemical bonding is important in many different functions o.
Ionic bondA type of chemical bond where two atoms or molecules are connected to each other by electrostatic attraction.