Proteins are polymers specifically polypeptides formed from sequences of amino acids the monomers of the polymer. A change in protein conformation produces a change in the net orientation of the dye relative to the surface plane and therefore the intensity of the second harmonic beam.
We have used nuclea.
What is the conformation of a protein. Proteins form by amino acids undergoing condensation reactions in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide. Protein farnesyltransferase catalyzes isoprenylation of the cysteine four residues from the C-terminus of several proteins including p21ras. Chromoproteins which contain pigments such as iron-porphyrins carotenoids bile pigments and melanin.
View How does a protein achieve its native conformationdocx from HUMANITIES 111 at University of Nairobi. PROTEIN STRUCTURE QUATERNARY describes the conformation assumed by multimeric proteins aggregates of more than one polypeptide chain. Conformation The precise shape of a protein or other macromolecule in three dimensions resulting from the spatial location of the atoms in the molecule.
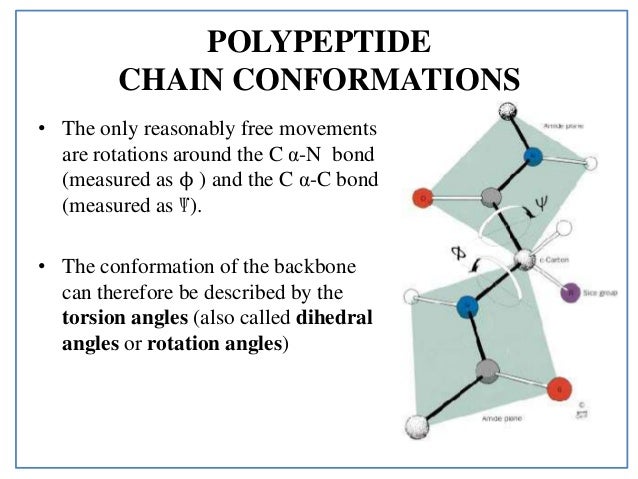
Describe the similarities between torsion angles and an energy vs torsion angle plot for the rotation of the C2-C3 torison angle with phipsi angles of peptide bonds and the 2D plots off allowed conformations around a given amino acid in a protein Ramachandran plot. A small change in the conformation s of some proteins affects their activity considerably. Macromolecules Section 1 - MALDI To F mass spectrometry Mass spectrometry is an analytical.
Farnesylation is required for the transforming activity of Ras and many efforts are underway to develop inhibitors of farnesyltransferase. Tertiary structure is largely maintained by disulfide bonds. The conformation of the protein arises from the bonding arrangements within its structure Figure 43.
Evolutionarily carriers are derived from the most primitive bacteria. 11 Single-Molecule FRET to Measure Protein Conformation. Protein conformation may be defined as the arrangement in space of its constituent atoms which determine the overall shape of the molecule.
A single amino acid monomer may also be called a residue indicating a repeating unit of a polymer. Disulfide bonds are formed between the side chains of cysteine by oxidation of two thiol groups SH to form a disulfide bond S-S also sometimes called a disulfide bridge. In other contexts the folded shape of a protein is most often referred to as its native conformation or structure Folded and unfolded proteins are often easily distinguished by virtue of their water solubilities as many proteins become insoluble on denaturation.
In a protein sample with a well-defined orientation the tilt angle of the probe can be quantitatively determined in real space and real time. The latter restriction differentiates conformation from configuration as in anomers and related stereoisomers where a bond or bonds must be broken in going from one form configuration to another. Protein Denaturation Drastic conformational change that destroys the function of a protein which occurs with extreme pH or heat and is most often permanent Factors Affecting Protein Action High temperature not low temperature pH too high or too low heavy metals can alter enzyme action.
Conformation of proteins in interfaces Like many other substances with both hydrophilic and hydrophobic groups soluble proteins tend to migrate into the interface between air. Conformation definition is - the act of conforming or producing conformity. The heat capacity of a native globular protein is close to the value found from the heat capacities of individual amino acid residues in an extended polypeptide chain.
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. The spatial arrangement of a molecule achieved by rotation of groups about single covalent bonds without breaking any covalent bonds. And finally nucleoproteins which contain nucleic acid.
Lipoproteins which contain lipids. How to use conformation in a sentence. Understanding how changes in protein conformation are linked to function is a key step in modeling molecular mechanisms for many enzymes.
Among experimental methods that measure protein conformational changes fluorescence resonance energy transfer Foerster resonance energy transfer FRET has proven to be of great use for sensitive detection of protein dynamics in physiologically relevant settings. Protein Conformation The characteristic 3-dimensional shape of a protein including the secondary supersecondary motifs tertiary domains and quaternary structure of the peptide chain. Conjugated proteins can be subdivided into mucoproteins which in addition to protein contain carbohydrate.
Different conformations are possible for any molecule in which a single covalent bond connects two polyatomic groups in each of which at least one atom does not lie along the axis of the single bond in question. Also for a protein composed of a single polypeptide molecule tertiary structure is the highest level of structure that is attained. Phosphoproteins which are rich in phosphate.